Key words: Analytical; analysis; chemical; chemistry; education; environment; environmental; instrumentation; monitoring; training; water; water quality



For advice on and training in scientific methods for environmental monitoring
Site still under construction
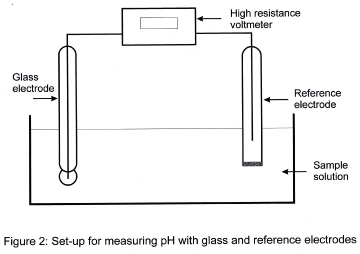
For the accurate determination of pH the use of a pH meter is the method of choice. There is a wide variety of such meters, ranging from small, portable instruments for use in the field to larger models which require mains power and so are laboratory-based instruments. However, all types are basically the same in that they involve potentiometry and so comprise an electrode which is sensitive to hydrogen ions, the working electrode, connected to a second electrode, the reference electrode, which is unaffected by any ions likely to be found in the environment. The two electrodes are connected through a high-resistance voltmeter which is calibrated in both pH units and millivolts. Modern instruments may also accept temperature probes so that measurements can be compensated for temperature.
2. The Glass Electrode
This is the working, or indicator, electrode. It is sensitive to the presence of hydrogen ions within the solutions in which it is immersed since it contains a fixed concentration of hydrogen ions (effectively 1.0 mol.dm-3) and a potential difference is generated between these and any external hydrogen ions. This potential difference which develops is proportional to the pH of the external solution.
The construction of a glass electrode is shown in figure 1 below.
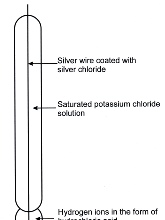
It can be seen that the electrode is in two sections. There is the glass bulb containing hydrochloric acid and above it a barrel containing potassium chloride solution. The two are connected by a single silver wire which is coated with silver chloride. Thus, the upper section functions as a silver-silver chloride electrode and its potential difference is determined by the potential set up between the hydrogen ion concentrations inside and outside the glass bulb when the electrode is immersed in a sample solution. This potential difference can be measured by connecting the glass electrode to the reference electrode, itself immersed in the same sample solution as figure 2 shows.
3. The Combination pH Electrode Unit
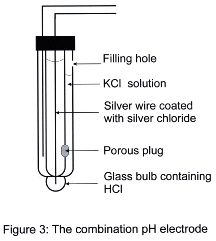
It is often inconvenient to have two separate electrodes immersed in the one sample solution and so the glass and reference electrodes are normally combined into one unit. This unit is known as a combination electrode. As figure 3 shows, it consists of two concentrically arranged barrels; the inner one constituting the glass electrode while the outer barrel forms the reference electrode. Continuity between the two electrodes is maintained through a porous plug in the wall of the glass electrode.
4. Calibrating a pH Meter
Although individual meters come with their own instructions all calibration procedures conform to a basic pattern. This is as follows:
Rinse the electrode unit and blot dry with soft tissue paper. The glass bulb is fragile so treat it carefully.
Insert the electrode into a suitable buffer solution (pH7.0 or pH4.0) and adjust the [CALIBRATION] control until the meter displays the value of the buffer.
Remove the electrode from the buffer solution, rinse with deionised water and blot dry with soft tissue.
Insert the electrode into a second buffer solution (pH10.0) and adjust the [SLOPE] control until the display reads “10.0.” The meter is now calibrated.